
43% of all athletes are dehydrated
When you exercise, you lose not only water, but also vital electrolytes and micronutrients. The consequences: cramps, headaches, and reduced performance.

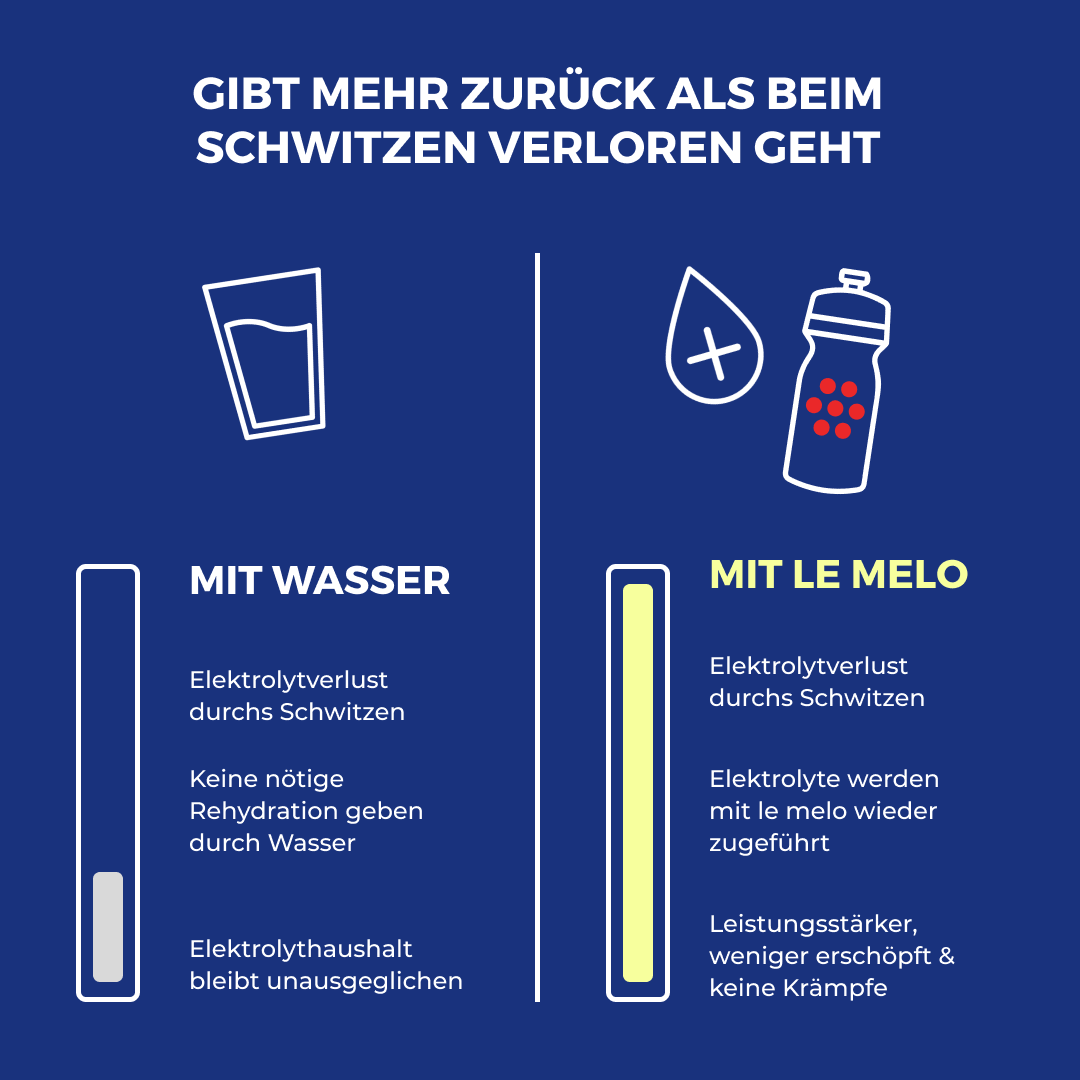
Did you know? Water alone is not enough
Water alone cannot replace your electrolytes.
The perfect sports drink requires:
- Electrolytes in a balanced composition
- Essential micronutrients
- The right balance of fast and long-lasting energy sources
- A formula tailored to your sport

More than just electrolytes
Our Hydration Core Formula delivers over 1000mg of electrolytes plus 13 essential micronutrients in every serving – far more than conventional sports drinks.
Scientific synergies instead of marketing overdose
Unlike many competing products, we don't rely on overdosing individual ingredients to show off impressive numbers on the packaging. We utilize scientifically proven synergies between nutrients:
- Calcium and magnesium in a 2:1 ratio for optimal absorption and effect
- Vitamin D, calcium and magnesium work together for better absorption
- Various magnesium compounds for both fast and sustained effects
Your power. Your choice.
Three functional formulas for every training goal and every requirement.
ALL ROUND
Your daily companion with a balanced blend of nutrients. Over 1000mg of electrolytes and 13 essential micronutrients ensure optimal hydration and well-being during moderate exercise. Perfect for fitness, running, and everyday training.
ENERGY
Your power boost for intense challenges. With natural caffeine from guarana and 8 B vitamins for sustained energy and mental focus – without the typical energy drink crash. Ideal for HIIT, competitions, and demanding workdays.
SUGAR FREE
Full performance with only 12 calories. Sugar-free, with 660mg of creatine for increased muscle strength and 250mg of L-carnitine to support fat metabolism. Complete electrolyte supply for those watching their calorie intake.







